NUSTAR - Stars and nuclei
Experiments with atomic nuclei are the key to understanding stars

We are all made of stardust. This is because stars and stellar explosions create the chemical elements of which our bodies and all living things are composed. Only the simplest element, hydrogen, was created exclusively during the Big Bang, which also generated much of the next-lightest element, helium, as well as significant traces of the lightest metal, lithium. To understand the stars, we first need to understand atomic nuclei. That is precisely what the scientists strive for with the experiments of the NUSTAR collaboration. They want to study the nuclear reactions that occur inside stars. This leads us to the world of exotic isotopes.
Exotic nuclei and the heavy elements
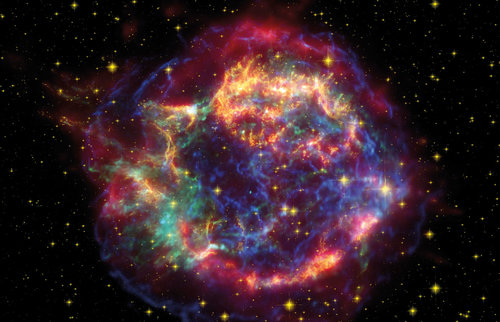
The Sun and the other stars shine because the atomic nuclei of light elements continuously fuse to create those of heavier elements. In this way, hydrogen creates all of the elements up to iron, the 26th element in the Periodic Table. This process creates energy in the form of heat and light — the sunlight and starlight we see here on Earth.
Heavier elements such as gold and lead are among the almost 70 elements that are created in stars and stellar explosions. They are generated by complex chains of reactions, which sometimes encompass hundreds of intermediate steps. Supernova explosions and mergers of neutron stars are important producers of these elements. These cataclysmic cosmic events generate many neutrons, which are captured by lighter atomic nuclei. However, the resulting neutron-rich nuclei are unstable and decay. In this process (known as beta decay), one of the neutrons in the nucleus is converted into a proton. The nucleus now contains an additional proton and has thus become the next-heavier element in the periodic system. Neutron capture and decay take place in a multiple and varying interplay of reactions. This process continues until a stable heavy element is created. However, everything happens very quickly. The atoms of all the heavy elements are created in the first few seconds after the stellar explosion.
The neutron-rich nuclei created, which are indispensable for the generation of the heavy elements, are different from the atomic nuclei found on Earth and may have completely different properties. Nevertheless, we would not exist without them.
The aim of the scientists at NUSTAR (Nuclear Structure, Astrophysics and Reactions) is to study the properties of these exotic nuclei. To this end, they are setting up several experimental stations that contain a variety of measuring devices. A key instrument that will be used for all of the experiments is the Super Fragment Separator (Super-FRS).
Sorting nuclides in the Super-FRS

The ions of the heaviest elements are shot at a target with a force that breaks them up into fragments on impact; these are the exotic nuclei of interest. However, they are extremely rare. The Super-FRS — a kind of sorting machine for nuclei — will help the scientists find what they are looking for. It is more than 100 metres long. With the help of magnets that weigh several tonnes, the system will sort the exotic nuclei according to their charge and mass. This will enable the scientists to filter out exactly the exotic nuclei they want to study — a feat not achievable anywhere else in the world.

