Nuclear Structure Physics
The chemical elements of the periodic table are the building blocks of matter and the basis of life itself. How did this variety of elements arise? What processes played a role here?
Answering these questions is one of the central concerns of modern nuclear physics and astrophysics, and also one of the main areas of research in the new FAIR-project.
We know that the chemical elements are formed through nuclear reactions inside stars and in stellar explosions. During this process, known as nucleosynthesis, a multitude of different types of nuclei, or isotopes, is formed. Most of these are unstable and decay into stable nuclei either directly or via several intermediate steps.
Fusion up to Iron
The elements up to iron are produced by fusion reactions inside stars. Beginning with the fusion of hydrogen into helium, larger and larger nuclei are formed. This process releases energy, which is the reason why the sun shines and provides us with heat. Fusion ceases with the element iron. This is because fusion into even larger nuclei would require energy input.
Detours to Uranium
Nuclei heavier than iron are produced at the end of the lives of large stars - so-called red giants - and in violent explosions of stars. All the production paths that occur in such circumstances lead to stable heavy nuclei indirectly via intermediate radioactive nuclei. The diversity of matter on Earth - and thus our existence - is due to a multitude of radioactive nuclei and nuclear reactions that happened in intermediate steps during nucleosynthesis.
To date, we have only a qualitative understanding of nucleosynthesis, the detailed processes are to a great extent still unknown. At the proposed new facility, scientists will be able to artificially produce and analyse the nuclei that occur as radioactive intermediate products in the formation of stable isotopes. The various processes involved in nucleosynthesis can thus be measured directly in the laboratory, and the intertwined paths of nucleosynthesis be traced. This will also permit a better understanding of the abundance of the elements in the universe. All of these nuclear and astrophysical aspects can be investigated in detail at the new facility. The project thus presents us with a fascinating view of the properties of nuclei and the origin of the elements - and hence of our own existence.
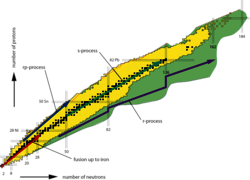
The chart of nuclides lists all the atomic nuclei that exist in the universe - over 6,000 in all - sorted according to the number of protons and neutrons. The black boxes indicate the stable isotopes (almost 300), the yellow boxes those unstable nuclei (about 2,500) about which at least something is known, and the green boxes the as yet unknown unstable nuclei (over 3,500 more). The arrows indicate various production paths of nucleosynthesis. Fusion produces nuclei up until iron. The most important production paths for the formation of heavy nuclei are slow neutron capture (s-process) and rapid neutron capture (r-process). In addition, there are other processes that lead to the proton-rich heavy nuclei. One of these is rapid proton capture (rp-process). The rp-process and the r-process run through areas far from the stable isotopes and can be investigated systematically and in detail at the new facility.